Research
We are focused on the pathology of inflammation in the colon, in the vessels, in wounds as well as systemic inflammation.
Molecules and biological mechanisms of our interest
Hylauronan
Cyanobacterial toxins and LPS
Microbiom and bacterial EVs
Extracellular vesicles as immune system signaling mediators
Shear stress in vessels
Thrombus formation and degradation pathology
Fibrosis and inflammation
Adenylate cyclases
Unique models in vitro and in vivo
In vitro
Model of intestinal epithelium
Model of blood vessels and microfluidic systems
Complex set of methods to test phagocyte functions, both granulocytes as well as macrophage
Complex set of methods to test T helper lymphocytes (Th cells) differentiation and functions, both mice and human
In vivo
Inflammatory bowel disease / colitis
Rheumatoid arthritis
Acute systemic inflammation
Therapeutical approaches
Therapeutics based on hyaluronan
Natural polysaccharides
Forskolin derivatives

Research Topics
Research Topics
ToggleHyaluronan in health and disease – development of novel therapeutics based on hyaluronan for treatment of inflammation-related disorders
Hyaluronan (HA), one of the body’s own biopolymers, is able to promote healing and modulate inflammation. The primary goal is to identify new mechanisms and principles of HA actions in the course of healing and inflammatory processes that will be used for development of HA based pharmaceuticals and biomaterials. This highly soluble and rapidly degradable polymer can be stabilized to enhance efficacy. Newly synthetized HA derivatives have acquired not only a range of physicochemical properties but also novel specific biological properties, they are able to modulate inflammation; acute in the case of acute wounds or surgical interventions; as well as chronic inflammation that often accompanies a variety of pathologies including non-healing wounds and intestinal inflammatory diseases.
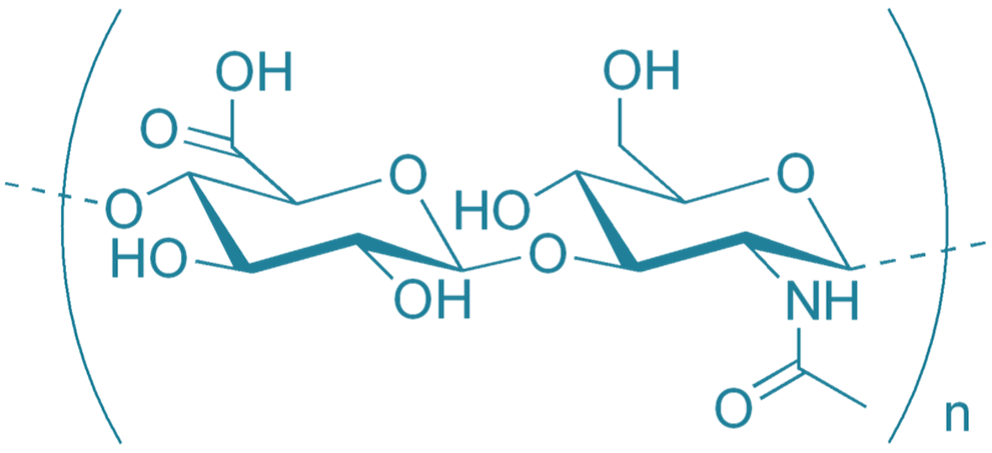
We focus on basic research related to HA regulatory functions, particularly on the effects of both endogenously produced and exogenously given HA on regulatory processes on the level of cells, tissue, and whole organism. We evaluate HA receptors, intracellular metabolism of glycosaminoglycans, and inflammation-mediated changes in synthesis and degradation of HA. We perform the determination of the pharmacokinetics of externally added HA using a unique methodology of stable isotope-labeled HA of different precisely defined molecular weight. In collaboration with the Contipro company, we used the obtained knowledge about HA derivatives for the development of new hyaluronan-based pharmaceuticals and biomaterials.
To screen the capacity of HA and its derivatives to modulate innate and specific immune responses, selected in vitro assays using human leukocytes or mouse leukocytes and lymphatic tissues are used. Furthermore, the efficacy and mechanism of action of the most promising candidates are studied in vivo. Our project should provide detailed information about effects of newly synthetized HA derivatives on myeloid cells, revealing their potential to affect inflammatory response and the pathological consequences, thereby helping to develop efficient state-of-the-art drugs and biomaterials in the treatment of inflammatory diseases.
Responsible person: Lukáš Kubala (Gabriela Ambrožová, Kristýna Turková)
Fibrosis and inflammation
Understanding of peritoneal fibrosis creation
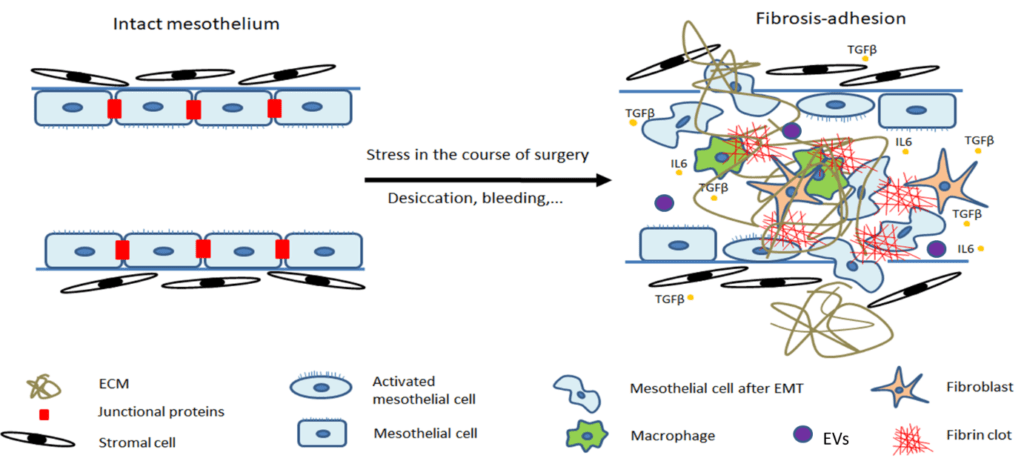
Within this project, we study peritoneal adhesions representing one of the major complications following intra-abdominal and pelvic surgery leading to symptoms such as abdominal pain, bowel obstruction and infertility. Inflammation is one of the crucial factors contributing to initiation and progression of fibrotic processes in peritoneum.
The communication between myeloid cells, mesothelial cells and fibroblasts is of fundamental importance in peritoneal fibrosis. Extracellular vesicles (EVs) are one of the communication channels. These membrane-derived particles can reflect their cellular origin and functional state through the bioactive cargoes they carry. We hypothesize that EVs together with soluble mediators represent crucial modulators in fibrogenesis and we aim to elucidate their role in the development of peritoneal adhesions.
Understanding the molecular mechanisms responsible for peritoneal adhesions development is necessary for implementation of effective strategies for prevention of this pathological process, including regulation of endogenous production of hyaluronan and hyaluronan-based antiadhesive barriers. Within our research, an innovative in vivo and in vitro models of peritoneal adhesions are implemented and modern molecular and biochemical research methods including flow cytometry and sorting, advanced microscopy, functional and metabolic assays are used.
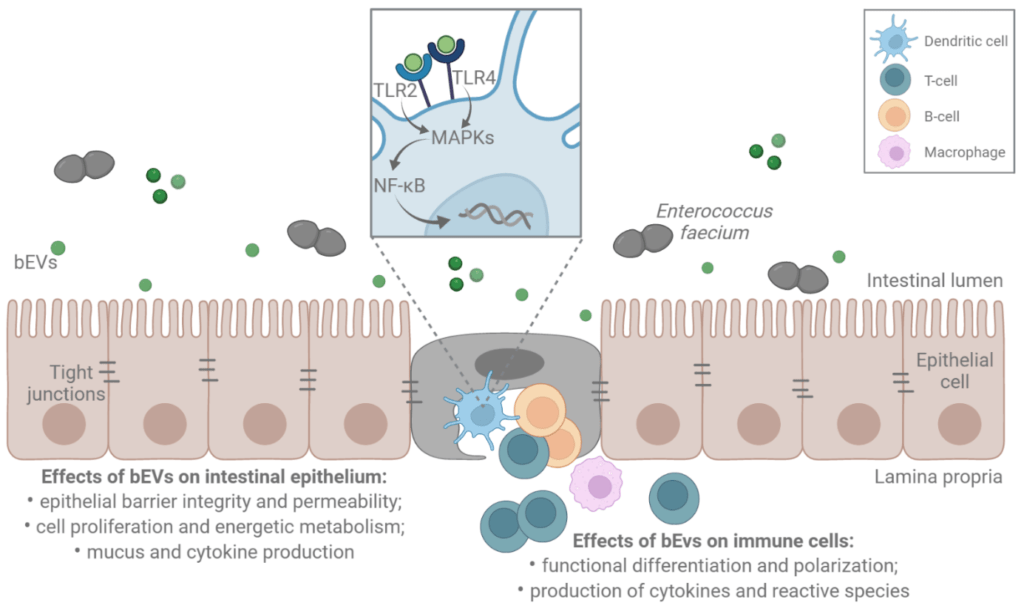
The role of bacteria-derived extracellular vesicles (bEVs) in chronic inflammatory bowel diseases
Chronic inflammatory bowel diseases, characterized by uncontrolled activation of intestinal immune cells in a genetically susceptible host, are undoubtedly one of the crucial pathologies afflicting modern society.
The main goal of the project is to characterize the composition and functional properties of gram-positive bacteria-derived extracellular vesicles (bEVs) and to reveal their role on the cells of intestinal epithelium as well as on myeloid cells participating in intestinal inflammation initiation and development. The key role of bEVs as a messenger delivering the information from the maternal bacterial cell to the host cell has been revealed recently and is also considered in the context of intestinal inflammation. However, bEVs origin, composition and proper mechanism of action on the intestinal epithelium as well as on relevant immune cell types remain unexplored, particularly in gram-positive bEVs.
The anaerobic cultivation in the anaerobic chamber is the crucial initial step for properly manipulating gastrointestinal bacterial cultures, followed by bEVs isolation, purification and characterization. Modern microbiological, molecular, biophysical and biochemical methods including flow cytometry, advanced microscopy, and functional and metabolic assays combined with bioinformatics approaches are used within project. Moreover, DSS-induced colitis in vivo model is used in a state-of-the-art animal facility localized at IBP CAS.
Responsible person: Gabriela Ambrožová, Kristýna Turková
Isoforms of adenylate cyclases as a potential therapeutic target
Cyclic AMP (cAMP) is an important intracellular second messenger, which is produced by adenylate cyclases (ACs). The known activator of membrane AC isoforms 1 to 8 is labdane diterpene forskolin. cAMP-regulated signaling pathways are recognized as important regulators of functions of cells involved in immune response and inflammation. In particular, three dominant AC isoforms (AC3, AC7, and AC9) are suggested. However, the importance of individual isoform on function of specific leukocyte subsets is not well understood. Thus, one of our aims is to clarify the importance of different AC isoforms in regulation of functions of selected leukocyte subpopulation (PMNLs, T- and B-cells) and to elucidate possibilities how to precisely modulate cAMP production in these cell types.
To improve knowledge regarding the regulation of different AC isoform activation we perform a comprehensive bioinformatics analysis of AC structures followed by analysis of structure by cryo-electron microscopy employing nanodisc technology. Next, we analyze the structural and functional consequences of selected clinically relevant mutations of ACs both by bioinformatics tolls and in the wet laboratory.
This research should provide a solid background for drug development based on modulation of specific AC isoform activity. This is done in close collaboration with medicinal chemists Assoc. prof. Kamil Paruch and Dr. Jakub Venda, from Faculty of Science at MU, Brno, who developed unique synthesis of forskolin that allows one to synthetize unique forskolin derivatives. We screen these newly synthesized derivatives for their potential to specifically modulate activation of a particular AC isoform.
Responsible person: Lukáš Kubala
Pathology of chronic inflammation-related diseases
In the project, we focus on two chronic inflammation-related diseases – rheumatoid arthritis and coronary artery disease.
Rheumatoid arthritis
Rheumatoid arthritis is a chronic inflammatory autoimmune disease caused by alteration of the innate and adaptive immune system. Currently, we investigate new polymeric delivery systems based on a water-soluble linear N- (2 hydroxypropyl) methacrylamide copolymer platform to improve therapeutic properties of the drugs carried either non-steroidal anti-inflammatory drugs (e.g. acetylic acid), steroids (e.g. dexamethasone) or biologicals (e.g. inhibitory antibodies against TNF-alpha – infliximab). The aim is to evaluate the biological properties and delivery of the drug to affected joints.
Coronary artery disease
Coronary artery disease, the most common cardiovascular disease, is related to a reduction of blood flow to the heart due to atherosclerotic changes in the arteries. Cardiovascular diseases remain the leading cause of death and morbidity in the industrialized world, with a rapidly increasing prevalence in developing countries. Chronic inflammation is a typical hallmark of cardiac disease, worsening outcomes irrespective of serum cholesterol levels. It is connected with multifactorial pathological processes involving various players, including endothelial cells, leukocytes, and platelets. The importance of selected mediators of inflammation including neutrophil phagocyte-derived myeloperoxidase is evaluated.
Responsible person: Lukáš Kubala
Physiology and pathology of shear stress in blood vessels
Vasculature is of extreme importance for maintenance of homeostasis throughout the whole body. Indeed, cardiovascular diseases are charged with the highest mortality burden in developed countries. Our focus is the connection between vascular inflammation and hemodynamics, thrombolysis in relation to ischemic stroke. Further we are interested in role of electrical gradients and currents in vascular homeostasis.
To understand many aspects of these phenomena, we are capitalizing on in vitro vasculature models. Fluidic models which range from simple chips with straight channels, to realistic, 3D patient data-based models are being optimized and utilized. To better understand aspects of ischemic stroke, static as well as fluidic models of thrombolysis have been introduced.
Vascular inflammation and hemodynamics
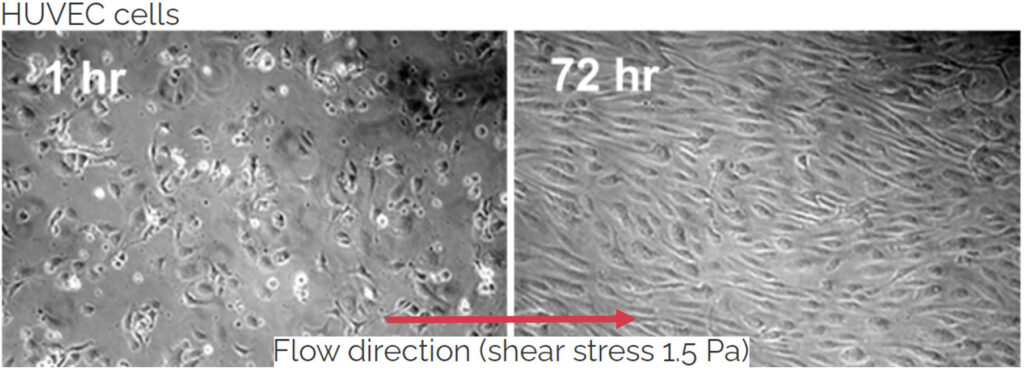
The vascular endothelium is a layer of specialized cell showing high degree of organization. The individual endothelial cell (EC) shows elongated shape mediated by highly organized actin filaments in the cytosol. Further ECs are oriented along with the blood flow. Tight junctions among cells mediate the primary function of vascular endothelium – regulation of vessel wall permeability which is under tight control and specific for a particular organ. Further the endothelial cells control vascular tone by means of sensing of shear stress (a coplanar force with the lumen of vessel wall which results from blood flow) and stimulating relaxation of smooth muscle cells in vessel wall by means of nitric oxide (NO) production. Importantly the endothelial cell layer regulates the immune response. Thus it is not surprising that the vascular endothelium is indispensable for homeostasis maintenance. Its dysfunction is a prerequisite for most of cardiovascular diseases including stroke and myocardial infarction. We are focused on studying the inflammation in microfluidic scale. On the other hand we employ 3D realistic models to study pathologies like intracranial aneurysms.
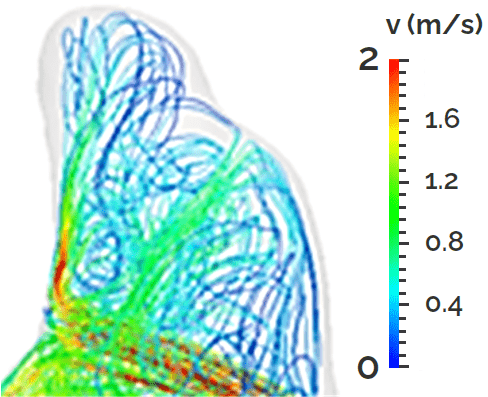

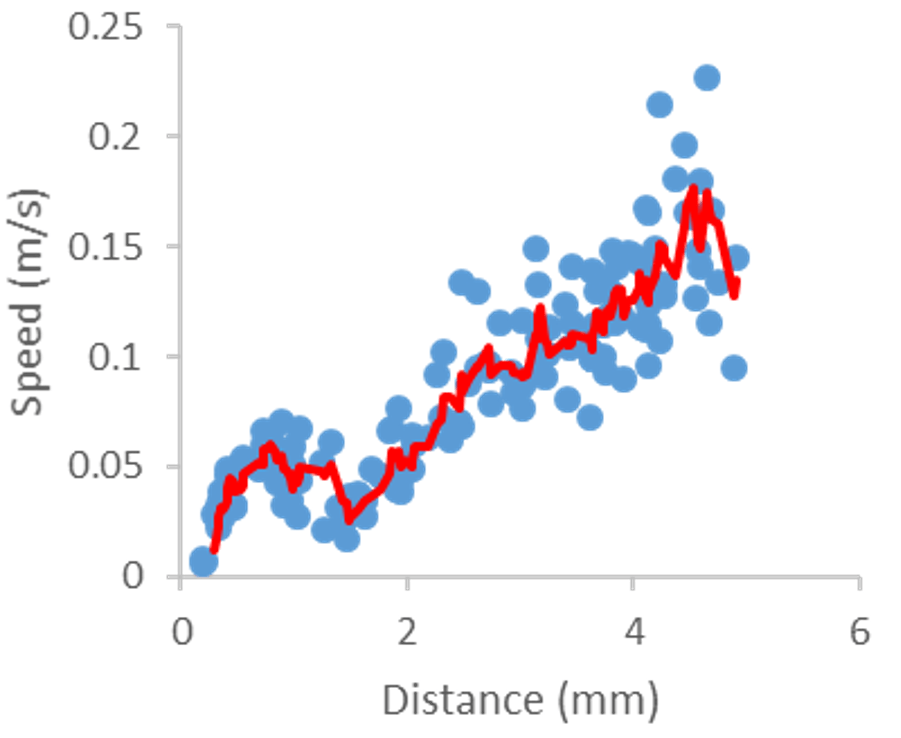
Comutational model of blood flow within an intracranial aneurysm (cooperation with Faculty of Mathematics and Physics, Charles University in Prague) and visualisation of the flow in laboratory model of such aneurysm together with evaluation of flow profile.
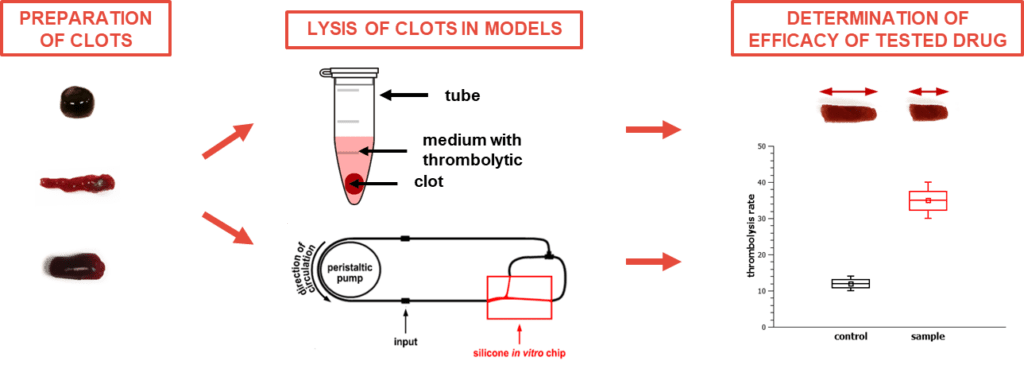
Thrombolysis in relation to ischemic stroke
Ischemic stroke is a disease with very high socioeconomic burden worldwide due to high incidence, mortality and morbidity. Ischemic stroke is caused by an occlusion of one or multiple cerebral arteries with a blood clot. Treatment priority in acute ischemic stroke is recanalization of such occluded artery. Currently, intravenous thrombolysis with a recombinant tissue plasminogen activator (rt-PA, alternatively known as alteplase) and/or endovascular therapy (mechanical thrombectomy) are the only clinically approved treatment options. Of those, who receive rt-PA, more than a half fail to respond to the drug. The factors behind limited efficacy of rt-PA are still poorly understood a complicate novel thrombolytic drug design.
Therefore, we focus on the in vitro modeling of events critical to vessel occlusion formation and thrombolysis or recanalization with a special emphasis of novel thrombolytic drug development. We have joined the extensive cooperation network covered by Stroke Brno research cluster, where multiple partners bring an extensive expertise and interdiciplinarity.
Electricity and vascular homeostasis
Fairly any tissue can produce as well as respond to electrical fields and ionic currents. This is of high importance in barrier forming tissue (epithelium and endothelium) which status is decisive for broad range of diseases (e.g. asthma, atherosclerosis, etc.). Intact cells in this tissue are tightly connected and form significant electrical gradients across the tissue. Once damaged the resulting ionic currents contribute to tissue repair. It has been shown that manipulation with such currents can modulate the tissue regeneration. The importance of membrane depolarization, calcium signaling, growth factors and morphogenesis pathways has been highlighted in the electrically mediate regeneration. The elastic modulus of the surrounding environment of cells is capable to modulate the regeneration as well.
That is why we employ electrodes made out of organic semiconductors. These materials show elasticity compliant to tissues and cells. Further, they combine electronic and ionic conductivity what is very important to interface living cells which show solely ionic conductivity. We cooperate with group of prof. Martin Weiter at Faculty of Chemistry at Brno, University of Technology who supports us with organic semiconductors and electronic devices.
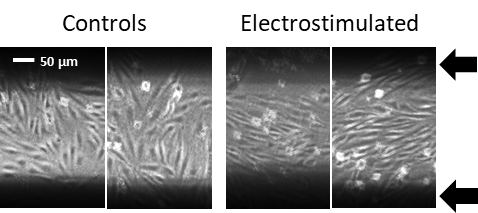
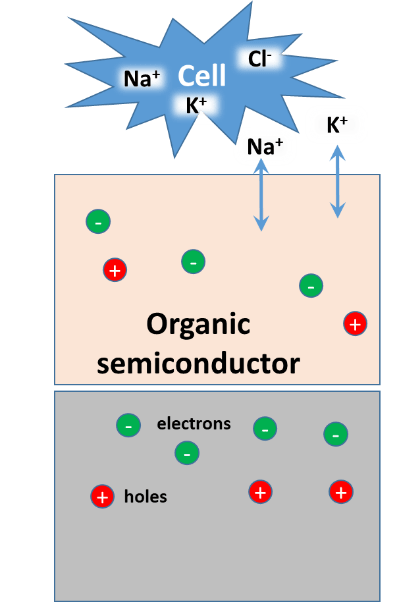
Responsible person: Jan Víteček
Mechanism of action of natural compounds on the immune response
Our group „Pharmacological regulation of the immune response“ focuses on understanding the mechanism of action of natural compounds such as polysaccharides from medicinal herbs, alkaloids or toxins from fungi and cyanobacteria on the immune response.
Pro-inflammatory and toxic effects of cyanobacterial secondary metabolites
The chemical diversity of cyanobacterial secondary metabolites by far exceeds our knowledge on their biological effects and their adverse effects on surrounding ecosystems including humans. On the top of the traditionally studied cyanobacterial toxins such as hepatotoxic microcystins or neurotoxic alkaloids anatoxin and saxitoxins, a large variety of structures exists, which possess biological activities making them relevant from ecotoxicological point of view.
Over the last two decades, at least two cases of novel cyanobacterial derived compounds have been associated with adverse effects and potential human toxicity. In the first case, the link between the exotic amino acid β-methylamino-L-alanine produced by cyanobacteria and amyotrophic lateral sclerosis-parkinsonism dementia complex has been proposed and established. In the second one, a novel cyanobacterial brominated toxin aetokthonotoxin has been found to cause vacuolar myelinopathy resulting in large mortalities of water associated biota with yet unclear toxicity in humans. Both these surveys also clearly show that except for the apparent adverse effects of cyanobacterial water blooms, also other cyanobacterial communities such as e.g. benthic, epiphytic and symbiotic can have toxicological relevance.
One of the chemical classes of cyanobacterial secondary metabolites calling for further toxicological studies are cyclic cyanobacterial lipopeptides. Puwainaphycins and minutissamides (PUW/MIN) are typical examples of cyclic cyanobacterial lipopeptides representing a broader class of widely-spread β-amino fatty acid containing lipopeptides such as e.g. muscotoxins, trichormamides and laxaphycins. They contain characteristic N-meAsp-Pro-(FA)-Val-Dhb motif in the vicinity of the FA moiety. While this region is conserved, the FA moiety and the peptide sequence on the opposite site of the cycle varies. PUW/MIN were shown to be generally cytotoxic in vitro causing cell membrane rupture and subsequent necrotic cell death.
One possible way for cyanotoxins to enter the body is through the intestinal tract. The small intestine is the major site for digestion, absorption, and metabolism of nutrients; it also responds to dietary signals. Most digestive, absorptive, and secretory processes are carried out by intestinal epithelial cells. The epithelium acts as a barrier against the entry of harmful agents such as pathogens, toxins, and foreign antigens. Homeostasis of the normal adult intestinal epithelium is maintained by continuous and rapid rejuvenation of differentiated cells by replication of undifferentiated epithelial or transit cells located within the crypts and subsequent differentiation of their progeny during migration away from the zone of replication.
Based on the available information, PUW/MIN may negatively affect intestinal tract homeostasis possibly leading to disruption of barrier function of the epithelium. Via increased permeability, other toxins, pathogens and so on may pass through. We study PUW/MIN in cooperation with Assoc. Prof. Pavel Babica, Dr. Ondřej Adamovský (both RECETOX, Masaryk University, Brno) and Dr. Pavel Hrouzek (Algatech Center, Institute of Microbiology AS CR, Třeboň). We are using state-of-the-art intestinal models such as organoids, 2D primary organoid-derived epithelial monolayer and 3D primary organoid-derived small intestine crypt model.
So far we have shown that non-toxic concentrations have pro-inflammatory effects on human intestinal cells in vitro and we continue to study the mechanism of their effect.
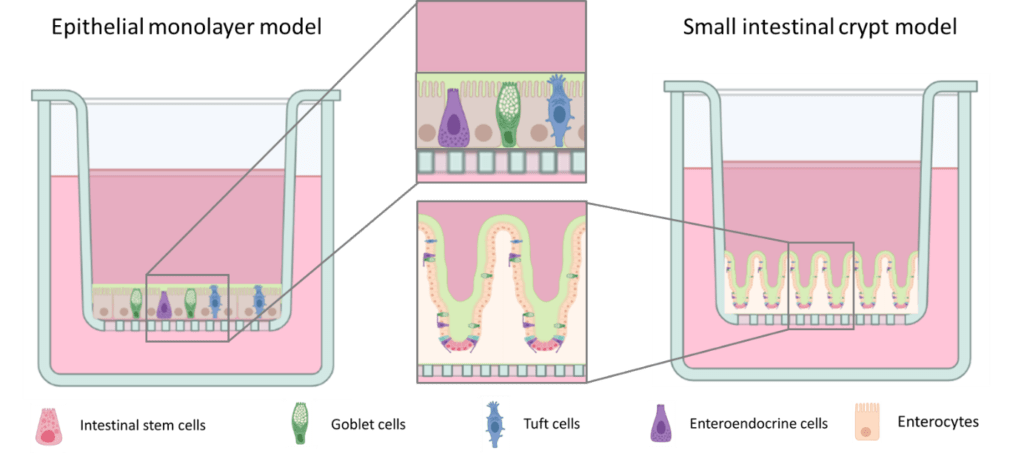
Responsible person: Ondřej Vašíček, Lenka Šindlerová
Investigation of immunomodulatory effects and biochemical mechanisms of immunomodulation with herbal and mushroom heteropolysaccharides
The investigation of the immunomodulatory activity of herbal and mushroom polysaccharides (PS) has enormous potential for the accumulation of essential knowledge with a real application in the development of products for medicine and dietetics. It is necessary to know the structure-activity relationship, in order to study the immunomodulatory effects and biochemical mechanisms of immunomodulation with PS.
From a structural point of view, the immunologically active herbal PS are predominantly presented by glucans (cellulose, other β-glucans, and starch), pectins, inulin and hemicelluloses (xylans, xyloglucans, arabinoxylans, galactoglucomanans etc.). Extremely popular sources of immunomodulatory and antitumor mushroom PS are Lentinus edodes (lentinan), Schizophyllum commune (schizophyllan), Trametes versicolor (PSK), Ganoderma lucidum and Grifola frondosa (D-fraction). It is important to be mentioned that mushroom research has been concentrated on Asian species, suggesting the prospect of screening studies on other widely consumed, but poorly-studied mushroom species.
The immunomodulatory activity of herbal and mushroom PS is expressed by complement-fixation, immunostimulating, anti-inflammatory, antiviral, anti-cancer activities etc. These activities are related to regulation of the metabolic activity of T- and B-lymphocytes, NK cells, neutrophils, macrophages, dendritic cells, platelets, as well as in suppression of inflammatory and tumor cells. The presented activities combined with the regulatory properties of PS on immune signaling proteins, complement system, antioxidant enzymes and probiotic bacteria may be useful for prevention and support therapy in infectious, tumor, gastrointestinal and autoimmune diseases.
Macrophages and neutrophils have a central role not only in our own immune defense, but also in the development of the response to immunomodulatory PS. Furthermore, PS regulate the production of inflammatory and anti-inflammatory cytokines by macrophages, depending on the situation, exhibiting immunostimulatory or anti-inflammatory effects. Although the PS effects on neutrophils are not as clear as in macrophages, it can be said that they increase neutrophil phagocytosis, bactericidal capacity, adhesion, superoxide dismutase activity etc.
It was found that herbal PS alone stimulated NO and ROS production by phagocytes but exhibited the opposite effect in a simulated microbial infection in our collaborative studies. It is hypothesized that in vitro, ex vivo or in vivo immunomodulatory effects of PS on macrophages and neutrophils via receptors may be useful against inflammation, viral infections, allergies, tumor, autoimmune and gastrointestinal diseases. This determines the interest in studying the biochemical bases of signal transduction in phagocyte immunomodulation with PS under normal and inflammatory conditions.
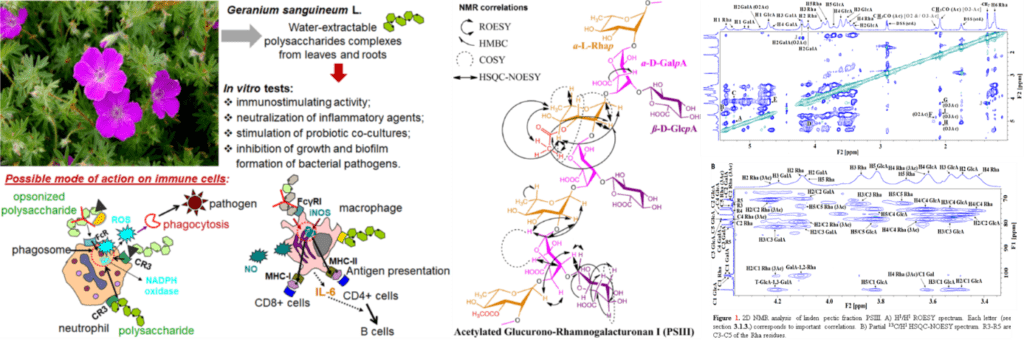
Responsible person: Ondřej Vašíček
Biomimetic Adjustable Hydrogel-based Scaffolds
The research and development of materials are crucial for progress in many scientific fields, including evaluation of the safety of various substances based on ex vivo reconstituted tissues. The creation of artificial intestinal tissue is very challenging, since its natural structure is very complicated.
The project therefore focuses on the preparation of materials mimicking the structural, mechanical, biochemical, and biological properties of individual layers of small intestinal tissue and on the preparation of scaffolds constructed from these materials. Hydrogel scaffolds employing gradients of extracellular matrix components and physical properties are cross-linked by biodegradable bonds. The scaffolds are then seeded with cells isolated from mouse small intestine. The combination of the structural, mechanical and biochemical properties of the scaffolds together with the application of external stimuli should ensure a cell instructive response, thus leading to the ex vivo formation of artificial small intestine tissue suitable for safety testing.
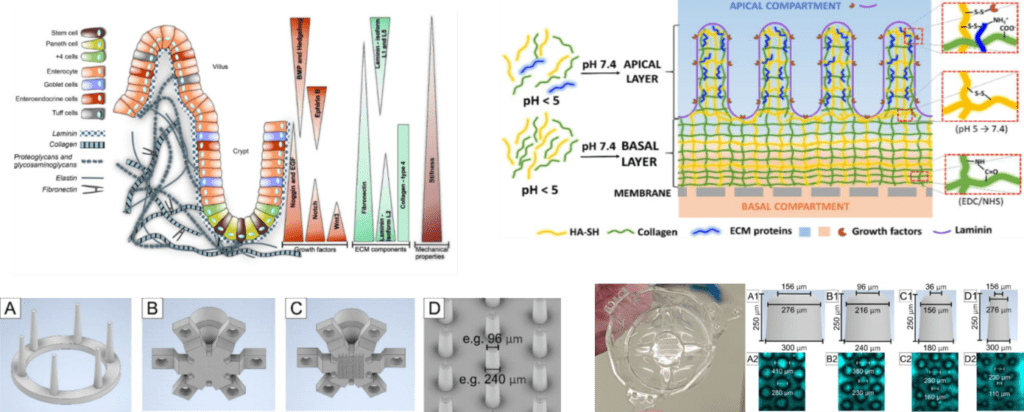
Responsible person: Ondřej Vašíček
Impact of cyanobacterial toxins on human health
We deal with toxic and immunomodulatory effects of natural substances and potential drugs. Our main direction at present are toxins from cyanobacteria dominating water blooms. Due to climate change, water blooms are becoming a global problem as not only their area of occurrence expands, but also their season extends. At the same time, new cyanotoxins are still being discovered.
Due to the fact that inflammatory reactions, such as eye irritation, skin irritation, gastroenteritis, etc., may be observed in sensitive individuals after recreational exposure, we pay particular attention to non-toxic immunomodulatory concentrations of these substances and look for mechanisms by which they act. These findings help to increase the accuracy of the risk assessment of environmental mixtures of cyanobacterial toxins for human health.
Relationship between structure and bioactivity of lipopolysaccharides of water bloom cyanobacteria
Cyanobacterial harmful blooms (cyanoHABs) are a serious problem worldwide. They consist of various cyanobacterial species associated also with heterotrophic bacteria. Cyanobacteria can produce a wide range of hazardous toxins which has attracted the attention of ecotoxicologists and during last twenty years many of them were discovered, described and mechanisms of their effects were clarified.
On the other hand, there are many others of which very little is known, even though they may be abundant. For example, lipopolysaccharides (LPS) represent a main part of the outer membrane of cyanobacteria and Gram negative (G–) bacteria. They can form up to several percent of dry weight of water bloom biomass, usually exceeding quantities of well-recognized cyanotoxins by one or even several orders of magnitude. Despite that, there is very limited information about toxicological effects of cyanobacterial LPS (cyanoLPS) and their health hazards.
Our research in the last years has shown that cyanoLPS can have pro-inflammatory effects in several cell mammalian/human types, i.e. immune or epithelial cells. However, the knowledge on the toxicity mechanism of bioactive cyanoLPS is still limited. Most importantly, their molecular targets as well as downstream proinflammatory signalling pathways appear to be very different from the action of prototypical LPS of G– bacteria. This can be likely connected to some specific structural properties which have been attributed to cyanoLPS in general, but the exact structures of most bioactive cyanoLPS from various bloom-forming cyanobacteria still need to be elucidated.
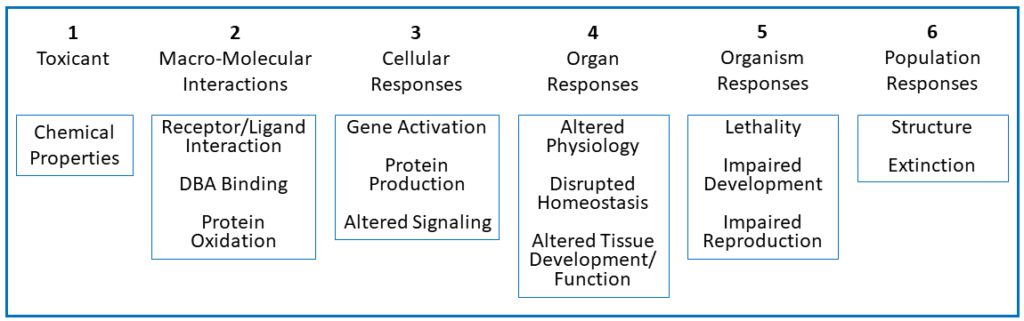
Knowledge of structure and the exact mechanism of action of environmentally relevant compounds (of natural as well as anthropogenic origin) allows for more accurate health hazard evaluation. Therefore, programme on development of Adverse Outcome Pathways (AOP) was launched by OECD in 2012. The aim of the AOP is to describe a sequential chain causally linked events at different levels of biological organisation leading to adverse health and ecotoxicological effects (Fig. 8). “AOPs are the central element of a toxicological knowledge framework being built to support chemical risk assessment based on mechanistic reasoning.” (https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm)
In this context, in cooperation with Assoc. Prof. Pavel Babica (RECETOX, Masaryk University, Brno) and Assist. Prof. Ondřej Jurček (Faculty of Pharmacy, Masaryk University, Brno) we aim to describe a hitherto unknown structure of selected active cyanoLPS and a precise mechanism of their effect, including an identification of their molecular target(s) and so-called molecular initiating event, the starting step of an AOP. These data will fill the gap in current knowledge on mechanistic understanding of proinflammatory activity of cyanoLPS, and help to complete inflammation-related AOPs, which should lead to improvement of health risk assessment of exposures to cyanoHABs.
Responsible person: Lenka Šindlerová
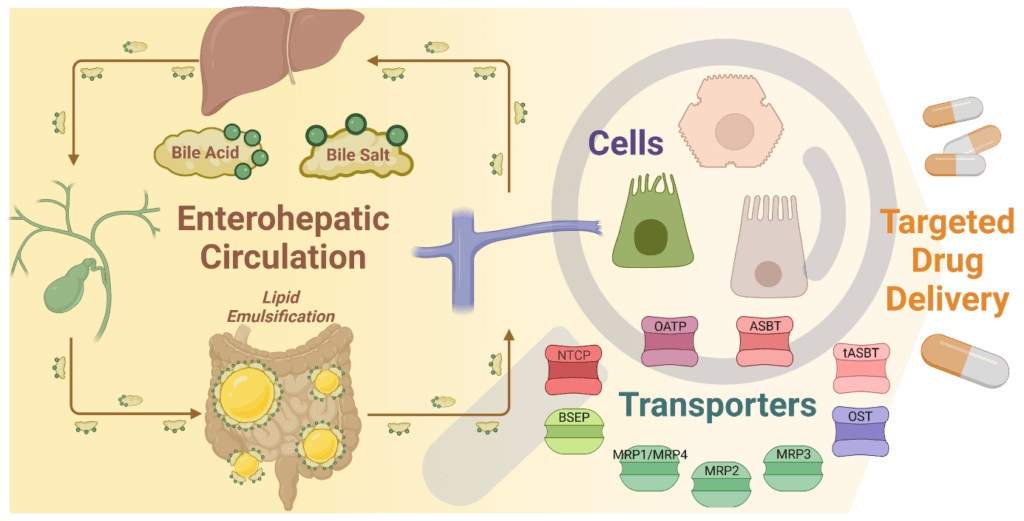
Development of Enterohepatic Drug Carriers: Porous Bile Acids-Based Metallo-Supramolecular Nanoparticles
Development of drug carriers represents an important area of pharmaceutical chemistry. One of the recent strategies studied is the use of porous nanoparticles of metal-organic frameworks. The use of porous solids for biomedical applications requires their biocompatible composition which is a significant limitation of development in the field. Bile acids (BAs) are natural transport molecules with high intestinal absorption and turnover within the enterohepatic circulation. The amphiphilic BA salts form mixed micelles with lipids and lipid soluble nutrients and transport them efficiently through intestinal wall.
We participate in project presuming that using BA-based coordination ligands in combination with essential metal salts will result in formation of biocompatible porous metal-organic nanoparticles – efficient drug carriers. The project is based on cooperation with Assist. Prof. Ondřej Jurček (Faculty of Pharmacy, Masaryk University, Brno) and Assoc. Prof. Pavel Babica (RECETOX, Masaryk University, Brno)
Responsible person: Lenka Šindlerová
