Intercellular communication in inflammation-connected pathologies

Responsible person: Gabriela Ambrožová, Kristýna Turková
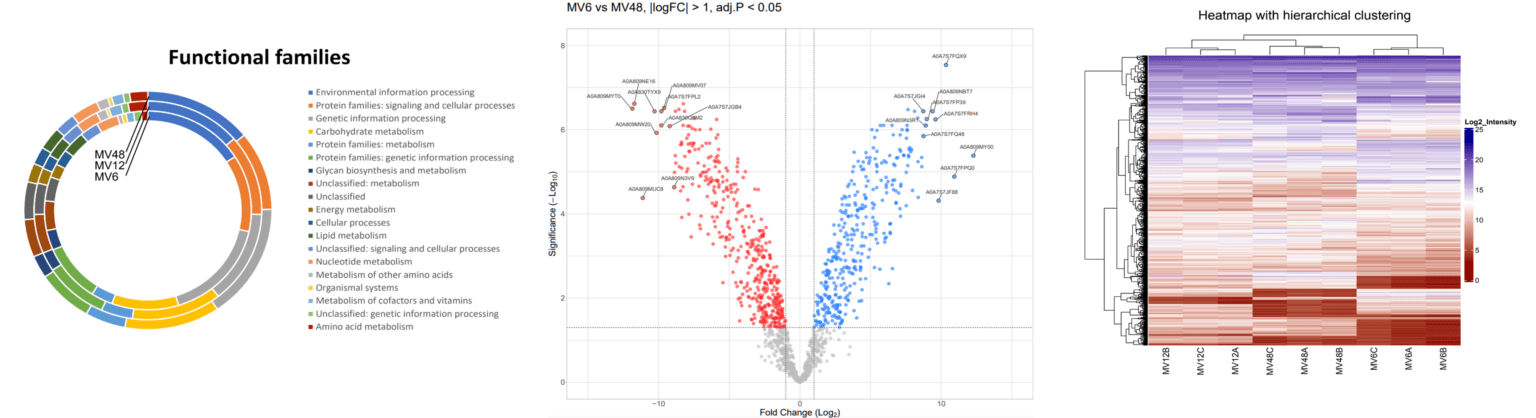
Intercellular communication is crucial in inflammation-connected pathologies, as it orchestrates the immune response and maintains tissue homeostasis; however, disruptions in these communication pathways can lead to chronic inflammation. The microbiome plays a pivotal role in inflammation-connected pathologies by influencing immune system function and maintaining gut barrier integrity. Dysbiosis, or an imbalance in the microbiome, can trigger inflammatory responses and contribute to the development of diseases such as inflammatory bowel diseases. Our research, emphasizing the dynamic interplay between the microbiome and host cells, aims to understand the molecular mechanisms of inflammatory processes in different tissues, revealing possibilities for their regulation. This understanding is necessary for the development of effective strategies for the prevention and therapy of diseases connected to chronic inflammation, such as inflammatory bowel diseases, cardiovascular diseases, arthritis, and peritoneal fibrosis.
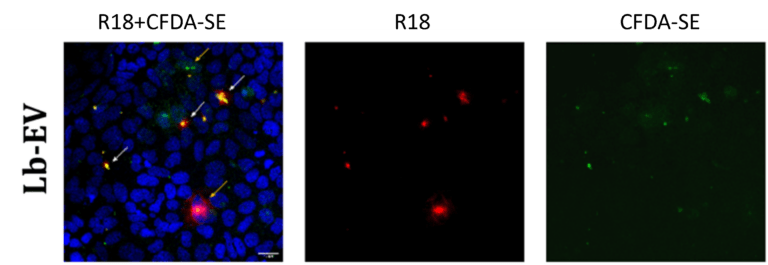
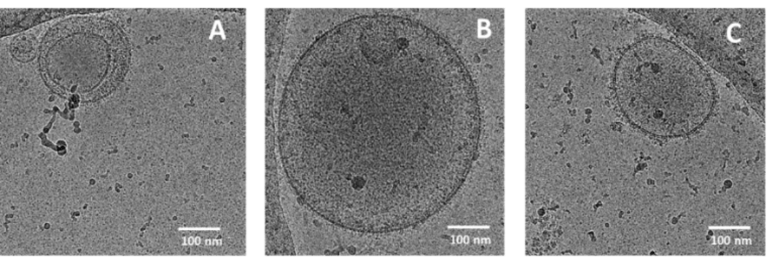
In studying intercellular communication, we focus on extracellular vesicles (EVs) of both eukaryotic and prokaryotic origin. These membrane-derived particles reflect their cellular origin and functional state through the bioactive cargoes they carry and can regulate inflammation. EVs, along with signaling molecules like cytokines and chemokines, facilitate the transfer of proteins, lipids, and RNA between cells, coordinating the activation, recruitment, and resolution of immune cells at sites of injury or infection. However, particularly the interaction of bacterial extracellular vesicles with immune host cells is poorly explored.
We employ innovative in vivo and in vitro models, alongside modern molecular and biochemical methods, including flow cytometry, advanced microscopy, and bioinformatics.
Our team is a member of the international group Czech Society for Extracellular Vesicles.